

|
|
|
#51
|
|||
|
|||
|
got it
|
|
#52
|
|||
|
|||
 Happy reefing. 
__________________
Randy Holmes-Farley |
|
#53
|
|||
|
|||
|
well the samples didn't arrive this week and i am going on vacation until the first of march . should be here by then : )
|
|
#54
|
|||
|
|||
 Have a nice vacation!
__________________
Randy Holmes-Farley |
|
#55
|
|||
|
|||
|
well i got back and the FMC is here but not the genchem.
this is the data sheet on the FMC. I will post any observations i might have and some pics in a couple hours : ) 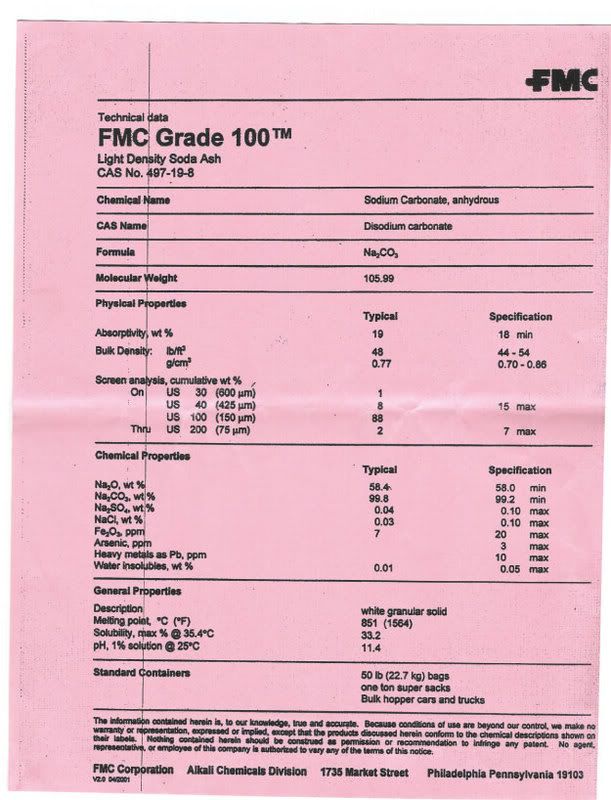
|
|
#56
|
|||
|
|||
|
That seems like a fine grade since it has arsenic and lead specs, and it likely fine to use. I see from their web site that grade 100 also meets drinking water specs. They sell many grades, so be sure what you are getting.

__________________
Randy Holmes-Farley |
|
#57
|
|||
|
|||
|
the sample i got was labeled FMC 100
From what he said . All the grades made at FMC meet the specs in the food codex. The only reason they can't call it food grade is because the plant itself doesn't qualify as a food grade plant. genchem makes one that is "food grade" However , when i talked to the salesmen there he told me that all the grades are so pure it would be a total waste of money to get the food grade version unless you were actually putting it in food and needed the certification. Again they all meet the food codex specs but only one is made in a food grade plant so it can get that certification. anyways it was substantially different than the arm and hammer version in several ways. First it came in much smaller granules , more like thin flakes and didn't have any soapy smell .  I baked some baking soda in the oven for 2 hours at 450 so i could make a batch of that to compare with. I mixed them up in one gallon jugs and then poured them into a clear container to take pictures. These two pics are of the baked baking soda   these two are of the ashed soda   First thing i noticed was this didn't turn the dirty brown color that the arm and hammer did or leave the brown film on top. The water is equally clear as the baked baking soda . It still did get some white foam on top . It also didn't smell like soap at all like the A&H did. It dissolves a ton easier than the baked baking soda. requires very little shaking to completely dissolve . It also generates more heat , the water doesn't get hot or anything but it certainly warms up. when you add the water it creates a immense amount of tiny air bubbles, so much that it fogs the water for a couple minutes , as these bubbles rise they create the foam on the surface. This foam disappears after a while. I wanted to see if the foam was like soap and would suds up if shaken so i pured it back into the jug and shook it really hard, the bubbles came back but only like 5% of them, nothing like when it was originally mixed. To wrap it up , this is absolutely different than the A&H washing soda. After mixing the A&H i would never have put that in my tank but I am considering using this, I haven't decided yet : ) I personally think baking the baking soda is the best idea right now but maybe this will turn out to be a good solution for people with larger high demand tanks. if the other grades ever get here I will let yas know if the results are any different : ) Last edited by Snarkys; 03/01/2007 at 05:23 PM. |
|
#58
|
|||
|
|||
|
wow great job keep us updated
|
|
#59
|
|||
|
|||
|
Randy: is it ok to make up 5gal of each of parts? I have the room for 2 5gallon buckets and this would last me a few months.
|
|
#60
|
|||
|
|||
|
Quote:
Sodium Bicarbonate - In aq soln it begins to break up into carbon dioxide and sodiumcarbonate at about 20C, and completely on boiling. HTH Last edited by palmerc; 03/02/2007 at 01:49 AM. |
|
#61
|
|||
|
|||
|
Quote:
|
|
#62
|
|||
|
|||
|
It will last forever in a closed container. Even if some CO2 entered or left it, it will still have the same potency and be fine to sue.

__________________
Randy Holmes-Farley |
|
#63
|
|||
|
|||
|
well i found someone that wants to be the ginnie pig : )
when the other samples get here i may give it a go also. |
|
#64
|
|||
|
|||
|
I am just about sold already... Sooo tired of baking.
|
|
#65
|
|||
|
|||
|
cant wait to hear this one out :P
|
|
#66
|
|||
|
|||
|
Quote:
I believe most of the commercial alk buffers are just a mix of sodium bicarbonate , sodium carbonate and a small amount borate. Guess it is possible the aquarium industry further purifies the sodium carbonate (soda ash) but i think that is doubtful. btw , i forget , why do they add the borate? |
|
#67
|
|||
|
|||
|
Borate is also alkalinity, but I think it does a good job of holding the ph because it's not depleted as carbonate is. Anyway we don't want it.
I forgot where on here it was, but it was a graph on the solubility of this stuff with temp. And it didn't seem to rise much after just a small ammount of heating. I've boiled the water in the past, but now I use a seio 2600 to mix a few gallons at a time. It mixes it perfectly without having to heat the water at all. I wonder if we can make a more concentrated mix using this along with a powerful mixing device. Look at B-ionic, it's 40% more concentrated and you can buy it at double the concentration and it is still a liquid. That would be cool. Especially since I've been adding 9 oz a day of each part latley. |
|
#68
|
|||
|
|||
|
the grade 100 dissolved almost instantly, required only a few seconds of shaking . FMC makes one that is even more absorptive than that. Possible you could get more into solution. Wish they had sent me larger samples. *EDIT* A local company is going to give me a 50# bag of the 100 to test with, I will see how much we can get into solution : )
http://www.fmcchemicals.com/TechData...9/Default.aspx Last edited by Snarkys; 03/06/2007 at 01:34 PM. |
|
#69
|
|||
|
|||
|
Just to make sure I've got this straight. Mixing baking soda with hot water (100 degrees Celsius) will achieve the same results as baking the baking soda first?
|
|
#70
|
|||
|
|||
|
well 3 cups of this soda ash 100 dissolved in a gallon of water immediately and 4 cups left just a few chunks on the bottom that may dissolve with bit more shaking . Let ya know what it looks like after sitting a day or so.
*EDIT* a bit more shaking and all four cups dissolved. one cup is 226 grams. Last edited by Snarkys; 03/06/2007 at 04:52 PM. |
|
#71
|
|||
|
|||
|
Cool, I had a feeling that it would. I asked Randy about it a while ago and he said they probably used better chemicals and better mixing equipment. I'm sure they are not baking arm and hammer. LOL
|
|
#72
|
|||
|
|||
|
So 900 grams for a gallon of solution... not bad. That may also help a lot with the ammount I have to keep on hand... The next step is to bump the calcium to the same concentration.
Snarkys I got your PM... I will take care of it in a day or so when I get some time. |
|
#73
|
|||
|
|||
|
Quote:
|
|
#74
|
|||
|
|||
|
I got way more than i need to test this stuff on my tank . I am happy to send some out for free to anyone that would like to try it. all i ask is you pay $5 for shipping. I think the small boxes i have around here will hold about two pounds.
just PM me for my paypal address. Please be aware while at face value it seems safe to use, it is in no way proven to be reef safe and by using it you are essentially being the ginnie pig. Please don't use it if you are uncomfortable with this. |
|
#75
|
|||
|
|||
|
If you mix ca(OH)2 with Na2CO3 in solution I would assume you would get a bunch of insoluble CaCo3 which common calk power.
__________________
for every question answered two more arise |
|
|