

|
|
|
#126
|
|||
|
|||
|
you can buy "soda ash", sodium carbonate, and sodium Bicarbonate at the pool stores... I have tried a buffer made from a mix of these, and it worked!
the thing is... it shows the "hazardous to all aquatic life" icon on the side of the sacks!
__________________
remember 10000 posts does not make you an expert... merely a saddo with no life except forums! |
|
#127
|
|||
|
|||
|
Quote:
the FMC 100 and the A&H washing soda are not the same thing. I know A&H says it is 100% pure but there is no way. if ya look earlier in this thread you can see pics of some I mixed up , it turns the water brown and has a brown sludge/film on the surface. It also smells like soap. There is no way i would use that washing soda in my tank. The FMC 100 mixes up clear like the baked baking soda does and doesn't smell like soap. |
|
#128
|
|||
|
|||
|
Quote:
I know this whole thing is all designed around DIY products to replace expensive store bought products but we should be careful and really think out some of the products that we use and don't get reckless about it : ) That said , I know that some pool maintenance companies around here stock the FMC 100 for internal use . I really think you are best getting this stuff in its original packaging from a manufacture that only makes soda ash. This really removes a ton of uncertainties about what you are getting and what you can feel comfortable recommending to others. Last edited by Snarkys; 04/01/2007 at 09:02 AM. |
|
#129
|
|||
|
|||
|
oh and the the sodium bicarbonate that pool stores have is almost always "feed" grade or lower . which means at best it is OK to feed to livestock on a farm but not humans . the difference in cost is so little and food grade is so readily available I don't believe it is worth trying to use these lower grades.
Last edited by Snarkys; 04/01/2007 at 09:05 AM. |
|
#130
|
|||
|
|||
|
Does one HAVE TO bake the baking soda before mixing? What if it's mixed unbaked and introduced to the tank?
thanks
__________________
"you work to live, you don't live to work" |
|
#131
|
|||
|
|||
|
Unbaked has a slight lowering effect on the pH of the tank. IMO the bigger problem is that you can only dissolve about half of what you could baked into the water. Even the baked version is 40% less concentrated than B-ionic, so most of us want to have the most concentrated mix we can.
I once asked Randy what he thinks they use to make B-ionic so concentrated and he said better chemicals. Remember that they also sell a double concentrated version that stays in liquid form. IMO it's got to have something to do with what's being discussed in this thread. |
|
#132
|
|||
|
|||
|
Boom:
I think A&H may have something else in it. It really smells like perfumed and is not as white or fine powdered so at least seems to be a different grade of sodium carbonate.
__________________
Did I write what I wrote? What the heck am I talking about! Well..... Nevermind. |
|
#133
|
|||
|
|||
|
Quote:
Actually you can dissolve 50% more Sodium Carbonate than what Randy's formula calls for the issue here is two fold. First. Baked by the user it is difficult to insure that all the bakers out there do a good job in beaking it properly so full conversion to carbonate is achieved and second the purity of the Calcium Chloride. Although you can also dissolve more Calcium chloride the idea is not concentrate it that much I assume in order to avoid concentrating other impurities and make it a little safer to use. (more fool proof if you like on both sides the alkalinity and the calcium part.)
__________________
Did I write what I wrote? What the heck am I talking about! Well..... Nevermind. |
|
#134
|
|||
|
|||
|
Yes I've had a problem just getting the 2 1/4 cups to dissolve when I first started. Now that I have refined my baking and mixing techniques it dissolves quickly and easily. This is baked at 500 degrees for over an hour with multiple stirrings to break up any clumps. Then I mix two gallons at a time in a bucket with a 2600 gph powerhead.
But I can understand what you are saying about the impurities. |
|
#135
|
|||
|
|||
|
Does it say Arm & Hammer Super Washing Soda on the box in big letters, I can't tell ? Many of us have used it in the past without this issue. We have been using this stuff for decades. I think you guys are dreaming or Snarkys got a bad box. I would not be making claims that it has additives when it is clearly stated what is in it.
 What are the ingredients in Super Washing Soda? ARM & HAMMER Super Washing Soda is 100% sodium carbonate. It does not contain fragrance, surfactants or other additives. How does Super Washing Soda differ from Baking Soda? ARM & HAMMER Super Washing Soda is 100% sodium carbonate and it is used as a laundry booster and general household cleaner. ARM & HAMMER Baking Soda is 100% sodium bicarbonate and has a myriad of household cleaning, personal care, and deodorizing uses, as well as being a leavening agent. IT is important to note that these two are two distinctly different products and cannot be substituted for one another. Last edited by Boomer; 04/01/2007 at 11:16 AM. |
|
#136
|
|||
|
|||
|
Yikes. I have been using A&H washing soda for about 6 months instead of baking the baking soda. I haven't seen any bad effects (acros are growing fine), but I don't like the sounds of it having additives.
Anyone know where you can get the 50lb bags of Gen Chem soda ash natural light, or the FMC product? Edit: was writing this before boomer posted. Are there different version fo the A&H product? (one with additives?) But still interested if you can buy the bulk bags of soda ash.
__________________
Greg Visit our CTARS club website by clicking the 'red house' icon above :) |
|
#137
|
|||
|
|||
|
Are there different version fo the A&H product?
Yes, at one time they had two and this has been an issue in the past with people buying the wrong one with additives. That is why I started positing pic of the box years ago. IIRC it was like Washing Soda and then Super Washing Soda
__________________
If you See Me Running You Better Catch-Up An explosion can be defined as a loud noise, accompanied by the sudden going away of things, from a place where they use to be. |
|
#138
|
|||
|
|||
|
I can only comment on my own experiences with it. It may very well have been a bad box but if the first box I got was bad then I would have serious concerns about quality control. The stuff is super cheap, I encourage anyone to buy some and mix it up to see if they get the same foggy brown water and the brown scum on the surface. Please smell it for perfumes also .
The box above is the product I used , it clearly states 100% sodium carbonate on the box. I contacted A&H and spoke with a few people about it, none of them had any intimate experience with the product but they all swore that the product was 100% sodium carbonate. judging by my own experience with this product VS the FMC 100 i have come to the conclusion that either they are flat out wrong, they use dirty equipment , equipment contaminated with other soapy products or the regulations on the soap industry are rather loose and 100% doesn't really mean 100% it may also mean 99.98% with the other 0.02% being perfumes and such. I spoke with the people at Gen Chem a few times on the topic . They supply A&H with the soda ash they repackage as A&H washing soda. They told me for absolute sure that the soda ash they send them is 100% sodium carbonate , however they can not comment on any additives they might add at the A&H factory before they package it or the cross contamination/cleanliness in the A&H factory. When I did RC searches for "washing AND soda" I also found a handful of threads where people had similar experiences to mine , brown water, soapy smell and some of them had negative reactions from using it in their tanks . There were very few if any first hand positive experiences using it. Again from my own experiences and research I personally feel much safer using product in FMC or Gen Chem factory packaging so i know exactly what I am getting. There is no way i would pour the brown mix A&H mix in my tank . The bulk 50# bags of factory product is prolly also drastically cheaper. Last edited by Snarkys; 04/01/2007 at 12:28 PM. |
|
#139
|
|||
|
|||
|
this is the A&Hwashing soda
 
|
|
#140
|
|||
|
|||
|
this is baked baking soda
 
|
|
#141
|
|||
|
|||
|
these are the FMC 100
  this is what it looks like dry 
|
|
#142
|
|||
|
|||
|
next time I am at the store I will by another box of A&H and see if the results are the same .
|
|
#143
|
|||
|
|||
|
Arm & Hammer has their own chemistry department. They have assayed stuff for me before, aquarium buffers and it has been posted here many times.
Have you ever contacted them. Not Arm & Hammer but Church and Dwight ? And when I say that I mean AH Specialty http://www.ahspecialty.com/
__________________
If you See Me Running You Better Catch-Up An explosion can be defined as a loud noise, accompanied by the sudden going away of things, from a place where they use to be. |
|
#144
|
|||
|
|||
|
Yes, I went back and looked at all those pic. It is obvious there is something wrong there.
__________________
If you See Me Running You Better Catch-Up An explosion can be defined as a loud noise, accompanied by the sudden going away of things, from a place where they use to be. |
|
#145
|
|||
|
|||
|
If my girlfriend didn't use it on the wash I may still have that box here , maybe i can try it again : )
I spoke with the people at Church and Dwight and their supplier Gen Chem. I wasn't able to get anyone in the lab or anything but the people i did speak with swore it was 100% . I have not visited that site before though. I really encourage other people to buy a box and see if they get the same results. I may have to go get one today and see if it is the same : ) |
|
#146
|
|||
|
|||
|
I haven't ever seen brown when I have mixed up A&H for dosing.
__________________
Greg Visit our CTARS club website by clicking the 'red house' icon above :) |
|
#147
|
|||
|
|||
|
I have had the same "brown" problem.
Here is my take. These products ARE NOT food grade and therefore not treated as such. They are not handled, shipped, packed, or stored as food grade material. I would not be suprised for a moment if A&H had NO FREAKIN IDEA what was in the product they package! It is very common to purchase raw materails from the lowest bulk bidder. You have no idea where they came from or how they were handled. You have no idea what "they" or "A&H" test for with each batch. You have NO IDEA what kind of QC program is in place to ensure the 100% purity. Like snarkys said. It is more than likely (almost a certainty) that the washing soda is boxed on the same line as the other powdered products that A&H sells or distributes. The recent DOG and CAT food problem is a perfect example. 100 or so companies were buying food and packaging it as their own. They were buying it from "menu foods" who was in turn buying bulk ingredients on the world market. I guess it turns out that one of the shipments of wheat protien was sent in a container ships cargo hold that was previously used to ship melamne. Contamination appears to be the cause. Food grade items are not supposed to be treated like this (but it still happens). Look into this some more and you may become scared to know how you food and other products are handled in this new "world economy". Look at the "Firestone" tire problems (The FORD rollovers) a few years back. The tire design was fine, the problem was in the fact that sidewall rubber and tread rubber are very different. Residual RAW tread rubber was still in the conveying equipment when it was time to make sidewall rubber. So the first hundred or so tires had tread rubber mixed in with sidwall rubber. The sidewalls were therefore less pliable and failed due to fatigue. The low tire pressure as recomended by FORD only made the problem worse. I can give (or you can find) dozens (hundreds?, thousands?) of examples of this. |
|
#148
|
|||
|
|||
|
Well we did use the rest of that box for laundry so i went and got a new box . I'd like to state these are only my observations take what you will from them. Anyone who wants to use any DIY chemical is highly advised to their own research and come to their own conclusions.
I used 64 degree RO/DI water and thoroughly cleaned the glass container . I did the FMC 100 first so if there was anything still in the jar it would show up in the FMC rather than the A&H. Both solutions got 2 cups of soda ash. First was the FMC 100 . It mixed in extremely fast . I think it took about 15 seconds to fully dissolve . It immediately creates an immense amount of micro bubbles that clouds the water and creates a foam as they rise to the surface. It had a smell but smelled like ozone , not like flowers or soap. it took about 3-4 minutes for all of the bubbles to surface and the water to clear. Here is a top and side shot. with and without flash . In person the water looks crystal clear , indistinguishable from plain tap water. (edit) the reason there is different amounts of foam on the top of these pics is because the first one without flash didn't come out well so i had to go back and take another)   Next I mixed up the AH.  First off i noticed nowhere on this box does it state that it is 100% sodium carbonate . I don't see it on the website anymore either . http://www.thelaundrybasket.com/Our_...shing_sod.html . It does say that it is 100% fragrance and phosphate free. I swear both the box and the website used to say 100% sodium carbonate , i really wish i had saved that box. This took a bit of work to dissolve, at least 3-4 minutes of mixing. When it did mix it also created a immense amount of bubbles that fogged the water but they took almost 40 minutes to dissipate so i could take a picture. This clearly smelled like soap or clean laundry.  This batch obviously came out much better than the last time I did it but it still had a brown tint to it. pics are shown with and without flash . I should say that the camera makes it worse than it is in person but there is clearly a brown tint to the water. The top to bottom are obviously worse because it is looking through twice as much water than through the side. 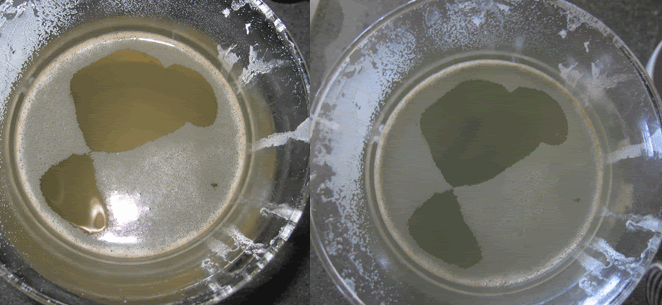 
Last edited by Snarkys; 04/01/2007 at 04:44 PM. |
|
#149
|
|||
|
|||
|
Good or bad , I think it's safe to say these are substantially different. The A&H takes more effort to mix, the bubbles linger 10 times as long, it smells like soap and doesn't result in the same water clarity.
I personally would not use the A&H but everyone should do their own research and come to their own conclusions . If anyone would like some of the FMC 100 to check against their A&H PM me and i will send ya a few pounds. It would be nice if you could post your own side by side pics to see if your results are the same. Last edited by Snarkys; 04/01/2007 at 04:59 PM. |
|
#150
|
|||
|
|||
|
Quote:
To be fair , i was just stating this as a possibility. I'm sure the legal standards for soap are not extremely stringent. |
|
|