

|
|
|
#1
|
|||
|
|||
|
Does this cartoon give accurate description of ph?
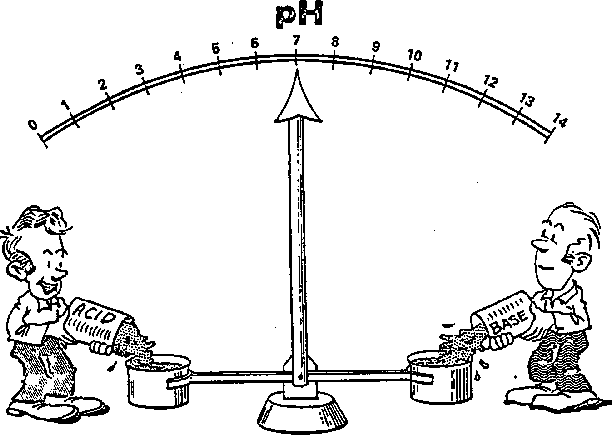 What do you think?
__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! |
|
#2
|
|||
|
|||
|
The scale goes slightly beyond 0 and 14, but other than that, it is basically correct.

__________________
Randy Holmes-Farley |
|
#3
|
|||
|
|||
|
So if theese guys keep adding bags of acid and base(in equal portions), what happens to water? Lets say they never stop adding it. Does the alkalinity raise up but stay at ph 7?
__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! |
|
#4
|
|||
|
|||
|
It gets very hot and salty, but nothing else happens if they are adding equal molar amounts of HCl (hydrochloric acid/muriatic acid) and NaOH (sodium hydroxide; lye).
There is no alkalinity remaining when they neutralize each other.
__________________
Randy Holmes-Farley |
|
#5
|
|||
|
|||
|
Hmm I find that interesting, and a good dipiction of ph. If the ph on the picture was lets say seawater chemistry (8.3 with 2.3alk) and they kept adding acids and buffers(in equal portions) the ph and the alkalinity of the water would stay the same?
Edidted So unless you add an acid or a buffer thats greater than a base or acid the ph will not change?(please dont include Co2 in this evaluation)
__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! Last edited by boxfishpooalot; 07/21/2005 at 03:56 PM. |
|
#6
|
|||
|
|||
|
Acids and bases in equal proportions will change only the salinity, not the alkalinity.
HCl + NaOH ---> Na+ + Cl- + H2O
__________________
Randy Holmes-Farley |
|
#7
|
|||
|
|||
|
So the ph stays the same at all ph levels, but gets saltyier. And the alkalinity changes only when one compound is added in excess of greater value (acid is more than base) Would make a more acidic ph/lower the ph.
__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! Last edited by boxfishpooalot; 07/21/2005 at 04:06 PM. |
|
#8
|
|||
|
|||
|
I have got a feeling that Randy might feel sorry that he said: it is basically correct.
 IMO, the cartoon only shows that pH 7 is neutral and above 7 it is basic and below 7 it is acidic. 
__________________
"I'm a big dumb stupid head." - Beerbutt Proud owner of the very rare YET (Yellow Elephantis Tang) from the Lord Bibah Islands. "LOL, well I have no brain apparently. " - dc (Debi) |
|
#9
|
|||
|
|||
|
Quote:
cool  Ps-sorry if I keep rambling on, and on. But Im really understanding alot from this and the difference in ph and alkalinity.
__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! |
|
#10
|
|||
|
|||
|
Strictly speaking, true buffers are always a combination of two or more compounds, one of which can take up protons at the pH of interest, and one of which can loose them.
So bicarbonate + carbonate is a buffer because HCO3- can loose one and CO3-- can take one up in the pH rang eof about 7.5 to 9 (in seawater). When you react a strong acid and strong base, the product is a salt and water.
__________________
Randy Holmes-Farley |
|
#11
|
|||
|
|||
|
Last one
 If we got a ph of 8.2 and alkalinity is 2.3(a mix of lets say bicarbonate and carbonate) to lower the ph you gotta add an acid. If I am right then I understand the difference in ph and alkalinity 
__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! |
|
#12
|
|||
|
|||
|
If we got a ph of 8.2 and alkalinity is 2.3(a mix of lets say bicarbonate and carbonate) to lower the ph you gotta add an acid.
In that circumstance, you can lower the pH by adding CO2 (carbonic acid) which will lower the pH but not the alkalinity, or you can add a mineral acid (like HCl) which will lower both the pH and the alkalinity. Likewise, you can raise the pH by removing CO2 from the system (which has no effect on alkalinity), or adding a base like sodium hydroxide (which will raise alkalinity and pH)
__________________
Randy Holmes-Farley |
|
#13
|
|||
|
|||
|
Well I think I got a handle on ph and alkalinity thanks Randy!
   
__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! |
|
#14
|
|||
|
|||
|
You're welcome.
Happy Reefing. 
__________________
Randy Holmes-Farley |
|
#15
|
|||
|
|||
|
I think the real question is where did you get the cartoon?
__________________
zen^2 |
|
#16
|
|||
|
|||
|
In Habib's new pH manual not yet published and in review

__________________
If you See Me Running You Better Catch-Up An explosion can be defined as a loud noise, accompanied by the sudden going away of things, from a place where they use to be. |
|
#17
|
|||
|
|||
|
ROFL, great thread
 
|
|
#18
|
|||
|
|||
|
Quote:

__________________
"You call someplace paradise, kiss it goodbye" The Last Resort, The Eagles |
|
#19
|
|||
|
|||
|
Yes, good guess , as he has a very acidic personality

__________________
If you See Me Running You Better Catch-Up An explosion can be defined as a loud noise, accompanied by the sudden going away of things, from a place where they use to be. |
|
#20
|
|||
|
|||
|
No neither one is me.
 Judging from the construction of the balance, it is not a logarithmic response. So it defintely is not me.
__________________
"I'm a big dumb stupid head." - Beerbutt Proud owner of the very rare YET (Yellow Elephantis Tang) from the Lord Bibah Islands. "LOL, well I have no brain apparently. " - dc (Debi) |
|
#21
|
|||
|
|||
|
You guys crack me up!

__________________
Its a good idea to have a refrence sample for alk test kits. 1.1350 grams of baking soda in 1gallon of distilled water=10dkh. Check your alkalinity test kit! |
|
#22
|
|||
|
|||
|
Yes Hab that is ture for sure, as you are a -log reponse

__________________
If you See Me Running You Better Catch-Up An explosion can be defined as a loud noise, accompanied by the sudden going away of things, from a place where they use to be. |
|
|